Gene Therapy for Parkinson's Disease and Other Neurological Disorders
We have been extensively involved in 10 gene therapy trials at UCSF since 2004. These include the AAV-Neurturin PD program (phase 1, 2 and 2b), AAV-AADC PD pipeline (phase 1, 1b, 1c and 2), pediatric AADC deficiency (phase 1), AAV-GDNF for PD (phase 1b) and AAV-miHTT for HD (phase 1/2). UCSF was the solo, lead or highest enrolling site in all of the trials completed to date, and we were directly involved in the development of the clinical protocols and surgical delivery methods used in all of these studies. By virtue of our participation in the planning and execution of these trials, we have become the most experienced center for intracranial gene therapy in the world.
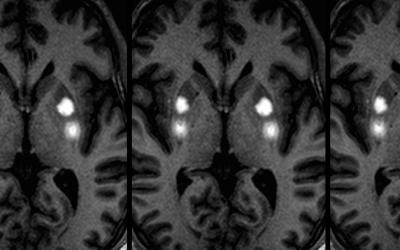
One major shortcoming in earlier trials was the inability to monitor infusions during the procedure to ensure adequate coverage of the intended target. In 2012, our group was awarded a grant to develop and perform phase 1b and 1c AAV-AADC clinical trial using a novel, real-time MRI-guided convection enhanced delivery (CED) technique with the ClearPoint system. This allows us to perform CED of viral vector mixed with an MRI contrast agent to visualize the infusions in the brain using real-time MRI imaging. These phase 1 studies allowed higher volumes of vector to be delivered with unprecedented efficiency in target coverage. A multi-center phase 2 trial using this technique, sponsored by Voyager Therapeutics, is currently enrolling subjects nationwide.
We are firm believers that MRI-guided CED is now the gold standard for drug delivery to the CNS. We are actively involved in gene therapy trials for other disorders using this delivery method, and techniques for cell delivery to the CNS are currently under development.
Key Team Members and Collaborators
Chadwick Christine, MD
Krys Bankiewicz, MD, PhD
Marin Thompson
Aaron Daley
John Bringas
Alastair Martin, PhD
Waldy San Sebastian Ramirez, PhD
Youngho Seo, PhD
Monica Volz
Nalin Gupta, MD, PhD
Michael Geschwind, MD, PhD
Related Publications
Marks WJ, Ostrem JL, Verhagen L, Starr PA, Larson PS, Bakay RA, Taylor R, Cahn-Weiner D, Stoessl AJ, Olanow CW, Bartus RT: Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson’s disease: an open-label, phase I trial. Lancet Neurol 7(5):400-8, 2008. Epub 2 Apr 2008. PMID: 18387850
Christine CW, Starr PA, Larson PS, Eberling JL, Jagust WJ, Hawkins R, VanBrocklin H, Wright JF, Bankiewicz K, Aminoff MJ. Safety and tolerability of putaminal AADC gene therapy for Parkinson’s disease. Neurology 73(20):1662-9, 2009. Epub 14 Oct 2009. PMID: 19828868
Marks WJ Jr, Bartus RT, Siffert J, Davis CS, Lozano A, Boulis N, Vitek J, Stacy M, Turner D, Verhagen L, Bakay R, Watts R, Guthrie B, Jankovic J, Simpson R, Tagliati M, Alterman R, Stern M, Baltuch G, Starr PA, Larson PS, Ostrem JL, Nutt J, Kieburtz K, Kordower JH, Olanow CW. Gene delivery of AAV2-neurturin for Parkinson’s disease: a double-blind, randomised, controlled trial. Lancet Neurol 9(12):1164-72, 2010. Epub 20 Oct 2010. PMID: 20970382
Olanow W, Bartus RT, Baumann TL, Factor S, Boulis N, Stacy M, Turner DA, Marks W, Larson P, Starr PA, Jankovic J, Simpson R, Watts R, Guthrie B, Poston K, Henderson JM, Stern M, Baltuch G, Goetz CG, Herzong C, Kordower JH, Alterman R, Lozano AM, Lang AE. Gene delivery of neurturin to putamen and substantia nigra in Parkinson disease: A double-blind, randomized, controlled trial. Ann Neurol 78(2):248-57, 2015. Epub 10 Jun 2015. PMID: 26061140
Christine C, Bankiewicz K, Van Laar A, Richardson R, Ravina B, Kells A, Brendon B, Martin A, Nutt J, Thompson M, Larson P. MRI-guided phase 1 trial of putaminal AADC gene therapy for Parkinson’s disease. Ann Neurol. Epub 25 Feb 2019. PMID 30802998
Richardson RM, Bankiewicz KS, Christine CW, Van Laar AD, Gross RE, Lonser R, Factor SA, Kostyk SK, Kells AP, Ravina B, Larson PS. Data-driven evolution of neurosurgical gene therapy delivery in Parkinson’s disease. J Neurol Neurosurg Psychiatry. Epub 30 July 2020. PMID 32732384

